How to handle and store Quarantine product and material
Quarantine register is structural format to recording failure product / material or deviation from the required specification in product information & tracking details.
Quarantine register is recording the information for the waste or unused material generate form the testing or manufacturing processes, in the process problems results are quarantine material are recording in quarantine register. In manufacturing processes in case any material being failure during the processes activities, or any mistakes on a process that cause failure of the product that became part of the quarantine system, some time the product’s deviation / variation are match with required product / material specifications are involved as failure of the product which goes to quarantine material storage, this material is storage separately in the manufacturing units due to avoid mixing with other product.
To maintain the product quality & processing system in the manufacturing unit, company is making the separate storage system where all the non confirmed products are storage to eliminate possibilities of the mixing, counting and analysis of the product failures, some time quarantine material is comes from the testing / laboratory divisions, reason that all the regular tested material, samples came from the customer for any further testing are collected, tested and after the result the sample are mostly not return if the customer had not indicated in the document, hence all the testing samples are also conducted as the quarantine material and storage in separately for the same.
Quarantine material is kind of waste from the quality testing or failure material that manage for system balance, quarantine material storage, handing and disposal are made in house that responsibility for that assigned by management to internal staff, but primary responsible for the declaration and in house settlement responsibility of quality department that all the tested samples and during the in-process samples are manage by quality so its should be declaration and send to quarantine material storage responsible is quality peoples.
When manufacturing processes, in processes in case nay product non conformity are found that product or materials are immediate identified for the quarantine for the separate storage if the immediate segregate. Manufacturing process owners are handing the process as and when required, the employee is identify and mark the failure material and given any identification number that indicate that lot numbers / batch number, process sequence numbers, machine and employee which indicates that material is identified as the quarantine, here below the example of the format of the quarantine log which is entered at the in process manufacturing:
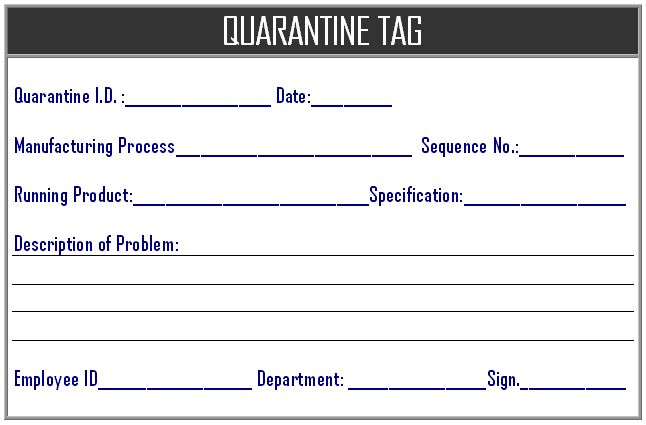
When employee is enter any identification number for quarantine material that should be consider the work order number along with identification number to mention “/” and work order number to easier for the tracking the material, in the quarantine log sheet should must include the failure information, identified problem to possible further investigation and analysis to prevent the error to avoid failure in product. Production manager / quality manager is decided for the storage or further analysis on the failure production material and after the disposal process is started for the proper storage and disposal as per instruction note, until the quarantine material is storage temporary at production areas with quarantine tag.
Quarantine record process
Quarantine record process is recording identified material from the manufacturing processes and all the material which is given for the storage at the quarantine storage areas, at the quarantine areas the records are maintain in the format is quarantine register which is established and maintained for the recording information, incoming and disposal quarantine material at quarantine storage area, here as below example given of quarantine register for reference:
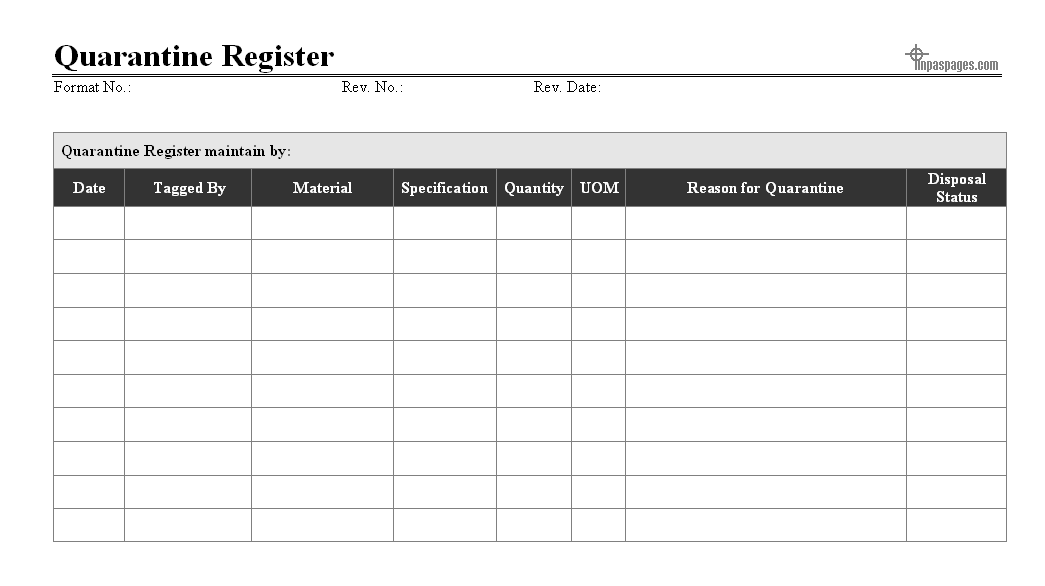
From manufacturing processes generated quarantine material is reach with quarantine storage area that all the documents are collected along with material, if the material inspected for checking defects that not required for further inspection, in case not that quality team can perform task if required in actions. All the incoming material at quarantine storage are recorded in quarantine register and also storage in charge is review the document and material for the primary inspection with reasons for quarantine segregated.
Quarantine register, quarantine log is maintain and disposal on base of the retention period, till than the register is helps to identification of the material which supporting the system of in-process analysis, investigation of the failure of product in manufacturing activities. All the quarantine material is dispose on base of the material specifications, government laws and to considering environmental issues with material disposal.
————————————————————————-
Download Formats In word Document | Excel Sheet | PDF Format
Download Quarantine Register in Word Document | PDF format
Download Quarantine tag in word document
————————————————————————-